

Traditionally, plate counts have been determined by manually counting colonies within 60-100mm Petri dishes.
#Imagej colony counting manual#
Manual Microbial Colony CountingĬompendial methods for microbial load and bioburden testing specify the determination of colony forming units (CFU) per gram of sample. Compliant with 21 CFR part 11 and GLP regulations when operated with BioCompliance™.īioSpot® Colony Counters vs.
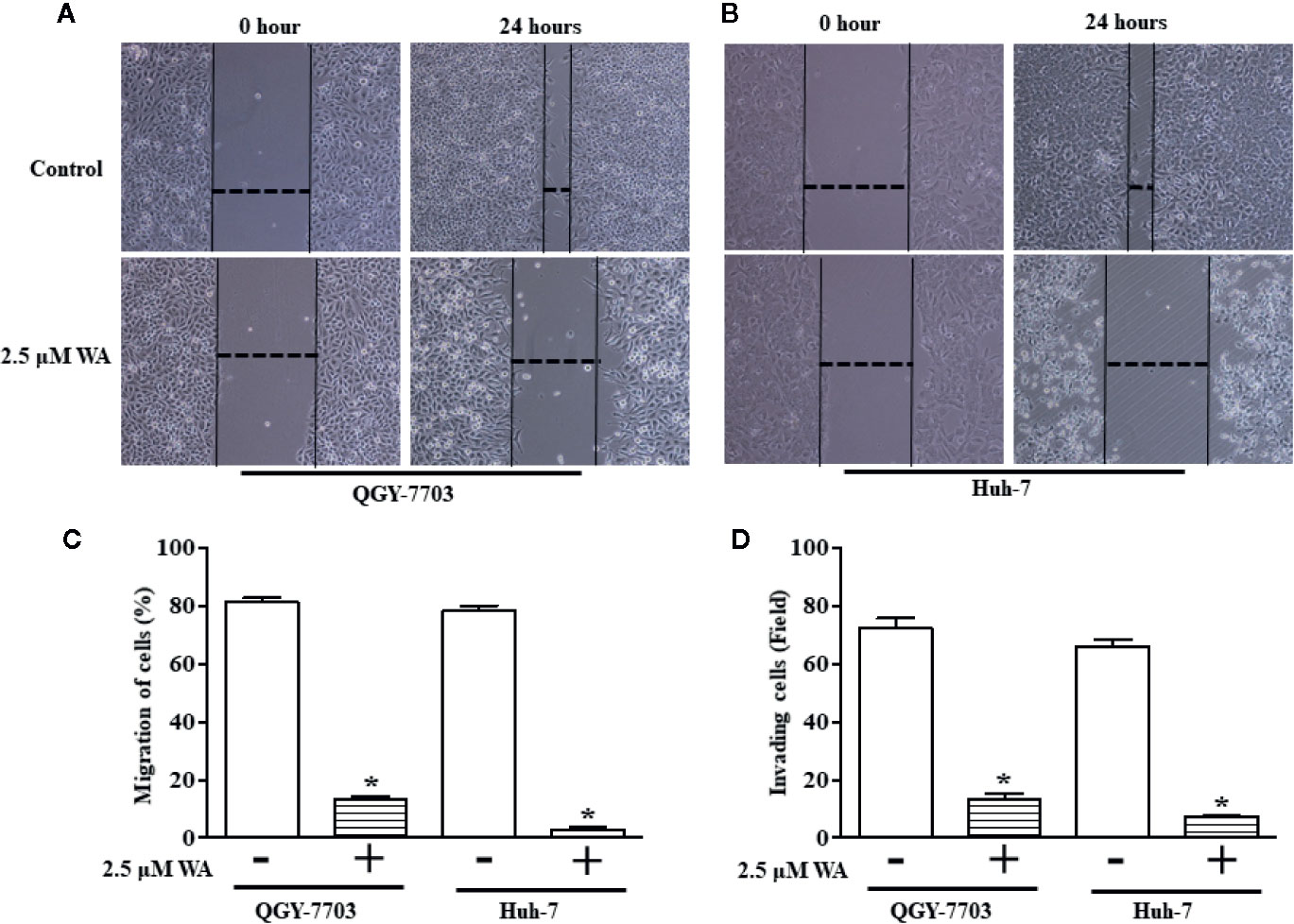
Ideally suited for rapid, high-throughput operations.Proprietary illumination enables detection of colonies as small as 25 micrometers in diameter.Compatible with all plate formats from 100mm Petri dish to 384-well microplates.These include microbial load and bioburden testing, clonogenic assays, stem cell assays, Ames test, mouse lymphoma assays, viral plaque assays, and more.
#Imagej colony counting software#
The BioSpot® line of instrumentation and software is highly adaptable and meets the needs of a broad range of colony counting applications. Analysis of the Effectiveness of Decontamination Fluids on the Level of Biological Contamination of Firefighter Suits.The CTL BioSpot® platform contains a wide range of automated image capture and analysis systems for microbial and mammalian colony formation assays.
#Imagej colony counting series#
Journal of Physics: Conference Series 2022, 2288 Application of image processing programs in color analysis of wood photodegradation. Cryogenic Laser Ablation in a Rapid Cooling Chamber Ensures Excellent Elemental Imaging in Fresh Biological Tissues. Yu Wang, Xing Wei, Jin-Hui Liu, Cheng-Xin Wu, Xuan Zhang, Ming-Li Chen, Jian-Hua Wang.Overall, this imaging-based method may greatly complement any signal-based pathogen-detection technique, especially in negating false signals, and therefore may significantly contribute to the field of analytical microbiology and biochemistry. The present method is sensitive enough to detect ∼100 bacterial CFU/mL but has the potential to estimate even lower concentrations with increased imaging and computation times. It could easily be tailored to estimate different species of pathogens, such as Escherichia coli O157, Listeria innocua, Staphylococcus aureus, Enterococcus faecalis, and Bacillus anthracis, on samples similar to those in real-time contamination scenarios. The method was validated against conventional microbiological techniques such as the use of optical density, flow cytometry, and quantitative real-time PCR (qPCR). Both the estimation and imaging parameters were optimized to reduce the estimation error ( E, %) to close to ±5%.
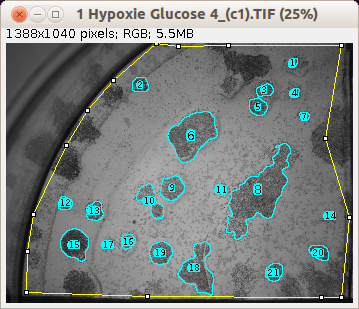
Herein, we explore a method that combines electron microscopy (EM) and image-analysis techniques and allows both visualization and quantification of pathogenic bacteria adherent even to complex nonuniform substrates. A visual quantification, therefore, may compliment such techniques by providing additional information and support better management decisions in the event of outbreaks. Conventional signal-based microanalytical techniques for estimating bacterial concentrations are often susceptible to false signals.


 0 kommentar(er)
0 kommentar(er)
